pee(k)
at the end of my time at USC, i was in a class where i was asked to come up with an idea for a startup and pitch it to prominent angel investors in LA. to date, this is the most interesting idea i’ve ever come up with. and yet, i’ve done nearly nothing with it. i’m hoping to use this piece to outline where i am and what i’m hoping to do next. let’s start:
the problem
i have a 76 year old grandmother who lives alone. she copes with it well, but sometimes she’ll forget simple things like going to a doctor’s appointments, eating the right healthy foods, or telling others when she’s showing signs of symptoms of being sick. this makes it hard on my mom and uncle, who have to check up on her every once in a while to make sure she is healthy and getting the proper care she needs. and it’s especially difficult because my mom lives in Indiana and my grandmother thousands of miles away in India.
my mom wants more frequent, up to date, and accurate health information about my grandmother than she’s currently getting through infrequent phone calls and WhatsApp messages.
the idea
that’s how i came up with pee(k): an internet-of-things (IOT) module that attaches to a toilet and analyzes one’s urine for symptoms of disease and general signs of health. this information is then made be available in a web and mobile application that you or those who care for you can use to track your health. pee(k) will even send recommendations, so you loved ones can improve their health over time.

the target audience
pee(k)‘s target users are senior citizens, but the target customers — those paying for the device — are caregivers to the elderly. these are unpaid individuals involved in assisting the elderly with activities of daily living or medical tasks. we’d specifically be looking at caregivers who are the children of the elderly.
this initial market is particularly interesting in that:
- a typical caregiver spends $12,700/year to provide care for an adult over the age of 50 if they live over an hour away from the care recipient. there’s a lot of money and time being spent here and everyone is aching for a better way to do things. source
- a UTI is the second most frequent infection in long-term care facilities. limited mobility among its residents makes it more difficult to gather urine samples to run frequent diagnostic tests. source
a potential business model
the total addressable caregiving market is 470 billion dollars in the US. there’s tremendous spending and investment here, which will continue to rise as the baby boomer generation needs more and more help. source
a business model approach i’ve been excited by is one that whoop has pioneered for specialized fitness devices: give the device for free and charge a monthly subscription for access to the data.
it is currently unclear exactly how much consumers would be willing to pay for this information (read: i need to do more surveys), but i believe pee(k) can charge a premium for the depth of data it would provide. let’s assume that is $30/month (equivalent to whoop’s price). further assuming the unit economics of the device is $100 and a consumer uses it for 2 years (akin to the time a user would keep their apple watch or fitbit), i would expect an LTV of $720.
the space
i started working on this 3 years ago, but paused as my time at USC ended. there’s been a lot of innovation since.
i view the space along two axis: target audience and depth of data output. unaliwear (raised $7.1 million) and carepredict (raised $19.7 million) stand out as companies that create fitbit-like devices for the elderly — meant to track their movement and not anything more. the others are far more interesting:
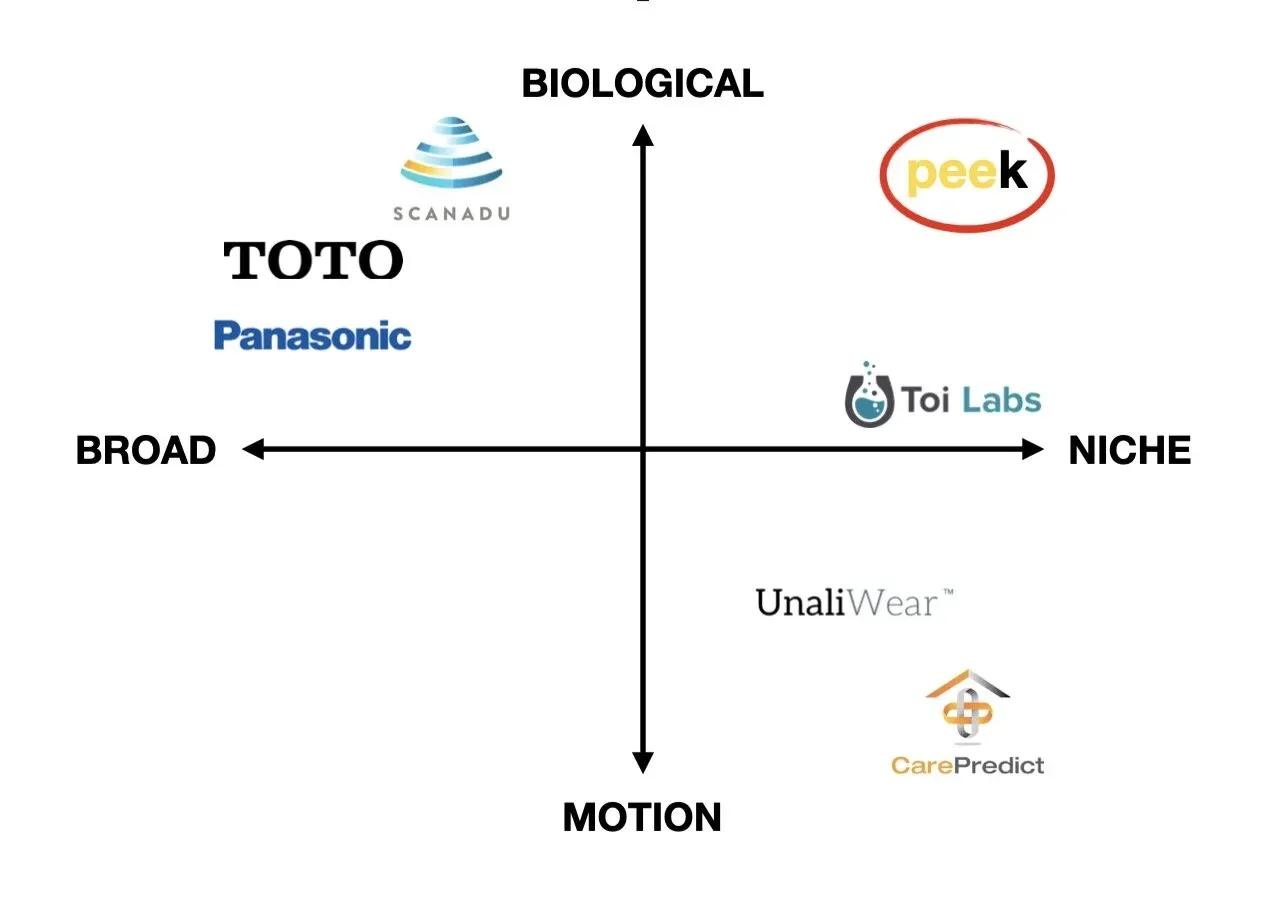
scanadu — it was a venture-backed private company which raised over $56,000,000. in 2014, it developed a urine test kit that enabled a consumer to analyze their urine with a dipstick and a phone. the test received FDA approval, but the company did a number of shady things and it went out of business. source scandal
toto — in 2005, toto, the world’s largest toilet manufacturer, launched their intelligence toilet. it was a toilet version of pee(k). for $3,500, anyone could have a toilet in their home that would analyze sugar levels, check blood pressure, body weight (from the toilet seat), and even hormonal balances. it never took off, although i believe this was from poor timing and high price, not a lack of innovation. the wearable trend and smartphones weren’t a thing yet — it would have been far more successful now. but for reasons mentioned earlier, a new toilet introduces more friction than is worth — i wonder how this would’ve performed as an attachable module. source
panasonic — in 2018, they also built an entire toilet to do all these fancy urinalysis things. the toilet also randomly included an armrest to measure a person’s body fat. and the flush included a fingerprint sensor to identify users. lots of cool concept ideas. on a random note, urine itself can be used as a digital fingerprint — no fingerprint sensor necessary. it’s unclear what has happened to this toilet since its initial press rounds. source
toi labs — this is the most similar company to pee(k) i could find. they’ve also focused on the older customer niche. their product, trueloo, takes a picture as you use your toilet to “analyze urine and stool for abnormalities in color, consistency, volume, and frequency.” neat. source
where i am today
i’ve started to validate the broad idea and nearly everyone i’ve spoken to has been fascinated and eager to learn more. but that’s it.
to date, i’ve done more speaking than taking action. there’s soundness in the idea, but does this solution actually work? there’s a lot that remains in assessing the viability of pee(k).
my challenges
urinalysis is a common test to perform and the easiest way to do so is with a dipstick test. the modern-day version of that requires a consumer to record a video of a small chemically-coated paper strip changing color as it reacts to their urine. this would be easy to use, but having to reload cartridges of paper strips voids the device’s ease of use. various universities have been developing lab-on-a-chip devices that can analyze urine for very specific conditions. while cheap to produce, it is unclear if multiple units can be placed together that each analyze for specific conditions, which can altogether beam their data for processing on a mobile device.
any sort of medical device like this — especially if it offers health recommendations, needs FDA approval. by building upon existing FDA-approved lab-on-a-chip’s, the cost of this may be reduced to $500,000. that’s a lot of frickin money. in the short-term, pending lawyer consultations, there may be a path where this device doesn’t provide the results of what it observes — instead, just indicating to its users “you might want to drink more water.” the nuances are tricky.
next steps
i’d like to have conversations with urologists, gastroenterologists, and the folks working on existing lab-on-a-chip devices. i’ve identified a few at Columbia University here in New York to start. by the end of May, i hope to have a much thorougher understanding of the procedures and technology at play here.
a faq
why would you make this for senior citizens? i can imagine so many more use cases!
i have no doubt in my mind there are far more use cases for such a device, but this entire concept needs more validation and seemed like the best place to start. the wearable trend, in particular, has popularized a notion of health and fitness metric tracking. pee(k) offers a key advantage in that users don’t need to remember to charge them or put them on. this would be a game-changer for nearly everyone who cares about their health. and for parents, pee(k) provides a non-intrusive to track their childrens’ health. for environmental scientists, pee(k) can offer insights into the type of waste flowing into our sewage systems. i’m certain there’s a longer list of use cases i haven’t even begun to think of.
a module? why not a toilet? why not a toilet seat?
when is the last time you purchased a new toilet? unless you recently remodeled your bathroom or purchased a new home, chances are your toilet has been around for a while. and even though replacing a toilet isn’t expensive, no part of the process is glamorous or easy. as a result, pee(k) must be an attachment to a toilet — it must be an extension that anyone can install.
why even toilets? and why urine?
no matter who you are, one of the things we have in common is our need for a toilet — beyonce and i both urinate; Queen Elizabeth and i both poop.
and yet, toilets have not evolved in centuries. in the United States, toilet-seat bidets are the hot new things as toilet paper vanishes off store shelves. the origins of bidets date back to the 1600s. the flush toilet was the hot new invention before then, assumed to have been invented in 1592. and since then, not much has happened.
the most recent widespread change in toilet technology may have been forced by the 1992 U.S. Energy Policy Act which required toilets use only 1.6 gallons of water per flush — a far cry from innovation. as a result, toilets became more efficient. the shape, the materials, the technology — nothing really changed.
what we put in toilets hasn’t changed either — it’s urine and fecal matter (and other products, but we’ll gloss over those). we flush away some of the most valuable information of our health and don’t think twice about it.
urine alone can be used to start diagnosing UTIs, health problems, kidney problems, diabetes, liver damage, blood disorders, and infections. for many of these abnormalities, a urinalysis test is not a diagnosis, but is a vital tool before more specific follow-up testing.
its less sexy cousin, feces, are strong indicators of digestive health. observing changes in their size, shape, and texture can reveal signs of infection, digestive issues, and cancer.
but fecal matter is chemically complicated and somewhat disgusting. with pee(k), starting with urine felt like the right idea.
other content on the topic
does this pee(k) (yes, i’m hilarious) your interest? if so, drop me a note.